With more than 60 years of measurement expertise, Keithley Instruments is a world leader in advanced electronic test instrumentation. Our customers are scientists and engineers in a wide range of research and industrial applications, including many electrochemistry tests. Keithley manufactures products that can source and measure current and voltage accurately. Electrochemistry disciplines that employ Keithley instrumentation include battery and energy storage, corrosion science, electrochemical deposition, organic electronics, photo-electrochemistry, material research, sensors, and semiconductor materials and devices. Table 1 lists some of the test methods and applications that employ Keithley products.
Table 1. Electrochemistry test methods and applications
| Methods and Measurement Capabilities | Applications |
| Cyclic Voltammetry | Electrochemical Sensors |
| Linear Sweep Voltammetry | Electrodeposition, Electroplating |
| Open Circuit Voltage | pH Measurements |
| Potentiometry | Solar Cells |
| Resistivity | Ion-Selective Electrodes |
| Current and Voltage Square Wave with Measure | Battery Charge/Discharge |
| Pulsed I-V | Semiconductor Device Characterization |
| Capacitance-Voltage | Electrochemical Etching |
| Source and Sink Current and Voltage | Corrosion Science |
| Measure DC Current and Voltage | Nano-Device Characterization |
Key Electrochemical Measurement Devices and Methods for Accurate Analysis
Cyclic Voltammetry
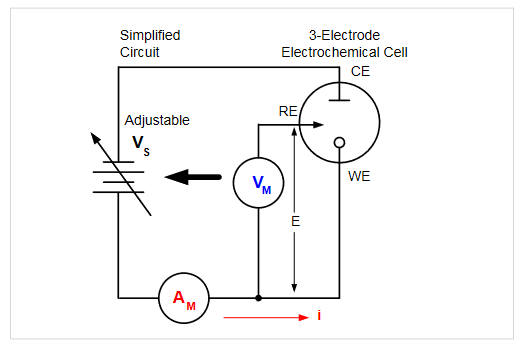
Cyclic voltammetry (CV), a type of potential sweep method, is the most commonly used electrochemical measurement technique, which typically uses a 3-electrode cell. Figure 1 illustrates a typical electrochemical measurement circuit made up of an electrochemical cell, an adjustable voltage source (VS), an ammeter (AM), and a voltmeter (VM). The three electrodes of the electrochemical cell are the working electrode (WE), the reference electrode (RE), and the counter electrode (CE). The voltage source (VS) for the potential scan is applied between the WE and CE. The potential (E) between the RE and WE is measured with the voltmeter, and the overall voltage (VS) is adjusted to maintain the desired potential at the WE with respect to the RE. The resulting current (i) flowing to or from the WE is measured with the ammeter (AM).
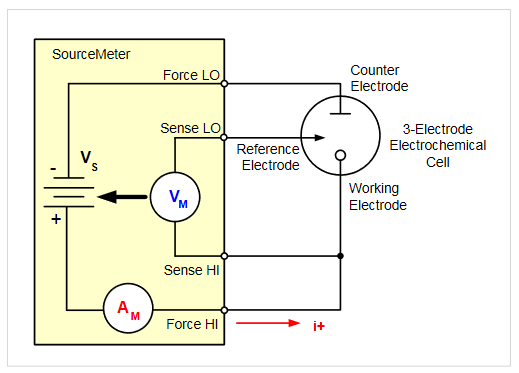
Keithley SourceMeter® SMU instruments source voltage and measure current, which makes them well suited for cyclic voltammetry applications. Figure 2 illustrates how the instrument's four terminals are connected to the 3-electrode electrochemical cell.
When a SourceMeter SMU instrument is programmed to source voltage in the remote sense (4-wire) configuration, internal sensing provides a feedback voltage that is measured and compared to the programmed level. The voltage source is adjusted until the feedback voltage equals the programmed voltage level. Remote sensing compensates for the voltage drop in the test leads and analyte, ensuring the programmed voltage level is delivered to the working electrode.
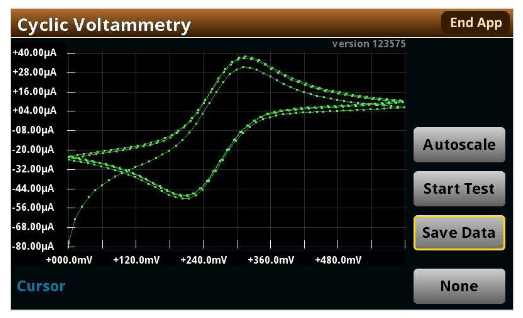
The 2450-EC, 2460-EC, and 2461-EC Electrochemistry Lab Systems have a built-in display that can automatically plot a voltammogram using its cyclic voltammetry test script. Figure 3 shows a voltammogram generated by the instrument. The 2450, 2460, and 2461 instruments include a test script that performs cyclic voltammetry without a computer.
Other electrochemistry test scripts that are included with the 2450-EC, 2460-EC, and 2461-EC Electrochemistry Lab Systems are open circuit potential measurements, potential pulse and square wave with current measurements, current pulse and square wave with potential measurements, chronoamperometry, and chronopotentiometry. These systems also include an electrochemistry translation cable with alligator clip set which enables the user to make easy connections between the instrument and an electrochemical cell.
Discover the full range of Keithley SMU products designed for cyclic voltammetry and unlock greater precision and efficiency in your electrochemical measurements.
Open Circuit Potential
The open circuit potential (OCP) of an electrochemical cell is a voltage measurement made between the reference and working electrodes. Measuring open circuit potential requires a voltmeter with high impedance to measure the voltage with no current or voltage applied to the cell. Because of their high input impedance, SourceMeter SMU instruments are well suited for OCP measurements when configured in a 4-wire configuration as shown in Figure 4. In this setup, the instrument is configured to measure voltage and source 0A. If OCP is measured before performing cyclic voltammetry, there is no need to rearrange any test leads manually between measurements because the instrument can automatically change functions internally. The 2450-EC, 2460-EC, and 2461-EC come with a test script for performing open circuit potential measurements.
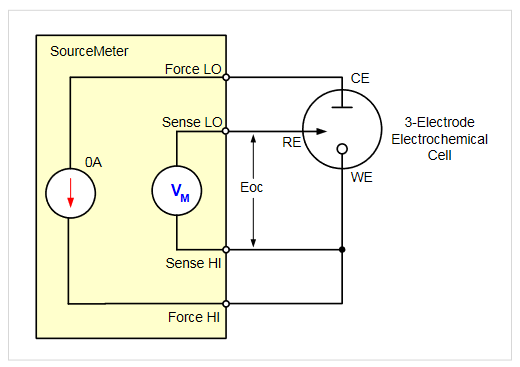
Visit our blog on how to use a Source Measure Unit to perform Open Circuit Potential of an electrochemical cell.
Resistivity
Electrical resistivity is a basic material property that quantifies a material's opposition to current flow. The best technique for determining the resistivity of a material will vary depending upon the type of material involved, the magnitude of the resistance, and the geometry of the sample.
Conductors and Semiconductors - Source Current and Measure Voltage
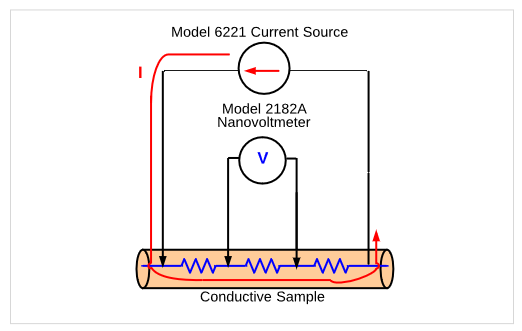
The resistivity of conductors or semiconductors is usually determined by sourcing current and measuring the potential across the sample in a 4-wire configuration. The 4-wire configuration minimizes the lead and contact resistance to reduce their effect on measurement accuracy. In this configuration (Figure 5), two leads are used to source the current and another set of leads are used to measure the voltage drop across a conductive sample. The voltage drop across the sample will be very small, so a Keithley 2182A nanovoltmeter is used to make the measurement.
Insulators - Apply Potential and Measure Leakage Current
An insulator's resistivity is typically measured by applying a potential to the unknown resistance and measuring the resulting leakage current. This is a 2-terminal test. The volume resistivity is a measure of the leakage current directly through a material. The surface resistivity is defined as the electrical resistance of the surface of an insulator. Figure 6 shows circuit diagrams for both volume and surface resistivity.
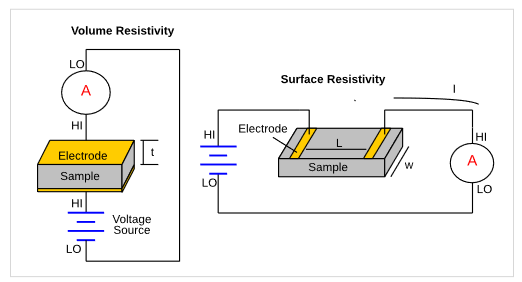
These high resistance measurements require an instrument that can measure very low current and apply a potential.Both the 6517B Electrometer/Voltage Source and 6487 Picoammeter/Voltage Source are capable of measuring the resistivity of high resistance materials. These instruments can measure currents as low as tens or hundreds of femtoamps and have a built-in voltage source. When measuring very high resistances, it is important to shield the device and all cabling properly to avoid the effects of electrostatic interference.
Visit this blog to learn more about Techniques for measuring resistivity for materials characterization using the 4200A-SCS Parameter Analyzer
Explore 4200A-SCS Parameter Analyzer
Potentiometry
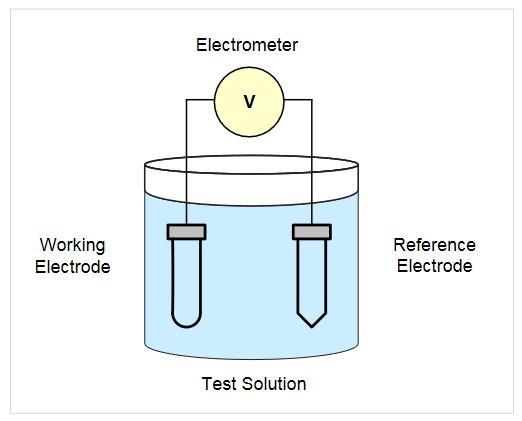
Potentiometry involves measuring the potential between two electrodes, typically a working electrode and a reference electrode. The potential difference is measured using a high impedance voltmeter or an electrometer, so that any current flow will be negligible (i=0). Potentiometry is used for applications such as pH measurements and voltage measurements made using ion selective electrodes. These potential measurements are usually made using two electrodes and a high impedance voltmeter, such as a 6517B or 6514 Electrometer (Figure 7).
Discover Keithley’s high-impedance electrometers for potentiometry applications
Electrochemical Sensors
Electrochemical sensors are used for many applications in diverse industries, including environmental and gas monitoring, medical applications like determining glucose concentration, as well as in the automotive and agricultural industries. Electrochemical sensors come in a wide range of differing designs; they may have two or three electrodes and could be potentiometric, amperometric, or voltammetric.Some sensors are based on organic electronic devices or nano-structures.
Choosing the optimum test equipment is crucial for electrochemical sensor R&D. For example, measuring the output of a potentiometric sensor may require a very high impedance voltmeter, such as a Keithley electrometer, which has high input impedance (>1014 ohms). Testing an amperometric gas sensor may demand the use of a very sensitive ammeter, such as a picoammeter, electrometer, or a SourceMeter SMU instrument.
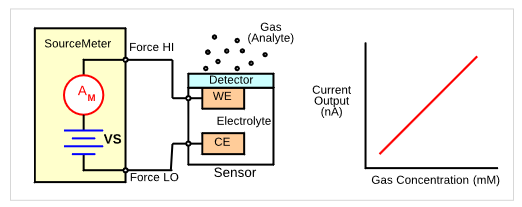
Figure 8 shows a simple amperometric gas sensor. When a gas comes in contact with the working electrode (WE) a chemical reaction occurs, either oxidation or reduction, depending on the sensor. In an amperometric sensor, current flows between the counter electrode (CE) and the working electrode (WE). The current output, which is related to the gas concentration, is measured by a sensitive ammeter. If necessary, a third electrode, a reference electrode, can be added to the sensor to apply a potential.
Discover test equipment best to use to characterize an electrochemical sensor
Solar Cells
To meet the increasing demand for clean energy, photovoltaic researchers are working to improve cell efficiency and reduce costs. Emerging technologies include dye-sensitized, organic, perovskite, and even 3D solar cells. Electrical characterization of solar cells is essential to determining how to make the cells as efficient as possible with minimal losses.
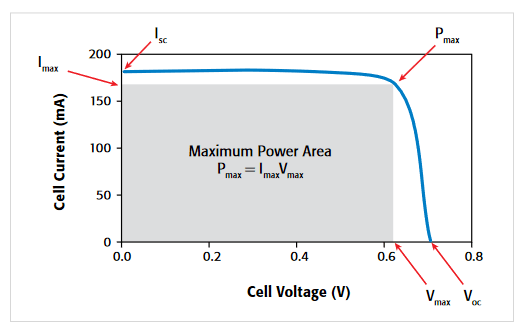
Some of the electrical tests commonly performed on solar cells involve measuring current and capacitance as a function of the applied DC voltage. Capacitance measurements are made as a function of frequency or AC voltage. Some tests require pulsed current-voltage measurements. These measurements are usually performed at various light intensities and temperatures. Important device parameters can be extracted from these measurements, including the output current, conversion efficiency, maximum power output, doping density, resistivity, etc. Figure 9 shows several parameters that can be extracted from a typical forward bias I-V curve on a solar cell, including the maximum current (Imax), short circuit current (Isc), maximum power (Pmax), maximum voltage (Vmax), and open circuit voltage (Voc).
Instrumentation like the 4200A-SCS Parameter Analyzer can simplify testing and analysis when making these critical electrical measurements. The 4200A-SCS is an integrated system that includes instruments for making DC and ultra-fast I-V and C-V measurements, as well as control software, graphics, and mathematical analysis capability. The 4200A-SCS can make a wide range of solar cell measurements, including DC and pulsed current-voltage, capacitance-voltage, capacitance-frequency, drive level capacitance profiling (DLCP), and four-probe resistivity.
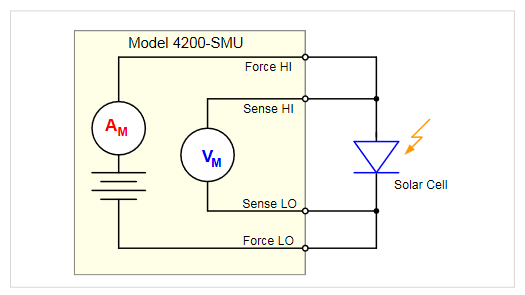
Figure 10 shows a solar cell connected to a 4200-SMU for I-V measurements. The four-wire connection eliminates lead resistance from the measurement circuit. Once the cell is connected to the output terminals, the 4200A-SCS’s interactive software makes it easy to set up voltage sweeps to generate I-V curves automatically, like the forward biased I-V curve of a photovoltaic cell shown in Figure 11.
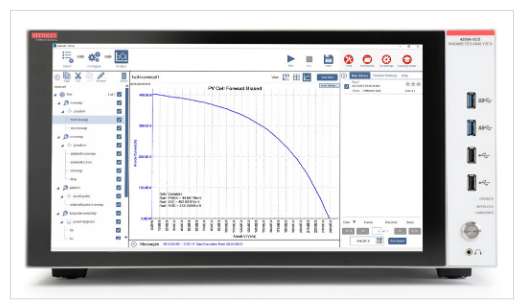
Learn about Electrical Characterization of Photovoltaic Materials and Solar Cells with the 4200A-SCS Parameter Analyzer
Explore 4200A-SCS Parameter Analyzer
Rechargeable Battery Charge/Discharge
Keithley SourceMeter SMU instruments can simplify battery testing because they’re capable of sourcing and measuring both current and voltage. These instruments have the flexibility to source and sink current as well as measure voltage and current, making them perfect solutions for battery charge and discharge cycling.
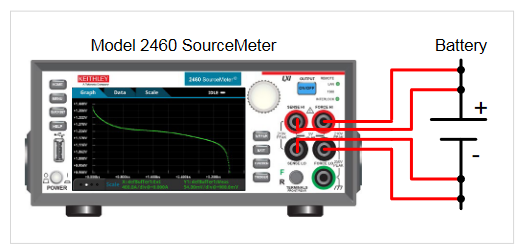
For this test, the SourceMeter SMU instrument’s terminals are connected to the battery (Figure 12) with a 4-wire connection to eliminate the effects of lead resistance.
For both charging and discharging cycles, the instrument is configured to source voltage and measure current. Even though the instrument is configured to source voltage, it will be in operating in a constant current mode. Figure 13 shows simplified circuit diagrams for both the charge and discharge cycles.
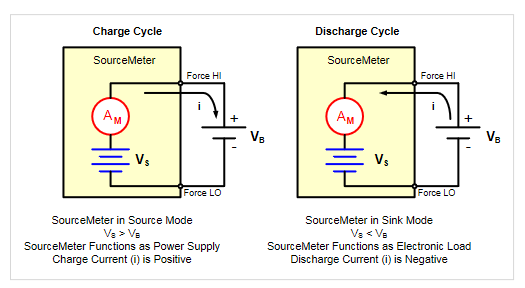
A battery is usually charged using a constant current, so the SourceMeter SMU instrument is configured to set the voltage source to the voltage rating of the battery and the source limit to the desired charging current. At the beginning of the test, the battery voltage is less than the instrument’s voltage output setting. This voltage difference drives a current that is immediately limited to the user-defined current limit. When in current limit, the instrument operates as a constant current source until it reaches the programmed voltage level.
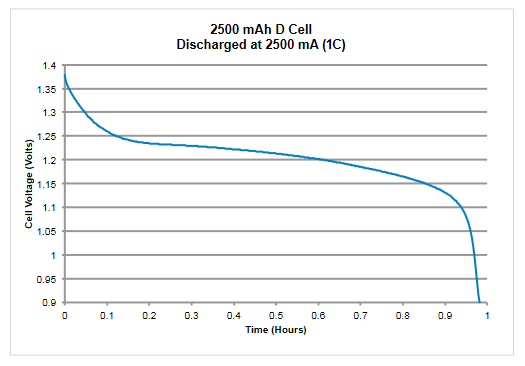
When discharging the battery, the SourceMeter SMU instrument operates as a sink because it is dissipating power rather than sourcing it. The instrument’s voltage source is set to a level lower than the battery voltage and the current limit sets the discharge rate. When the output is enabled, the current from the battery flows into the Hi terminal of the Instrument. As a result, the current readings will be negative. The results of measuring the discharge characteristics of a 2500 mAh battery are shown in Figure 14.
Learn about our guide on Rechargeable Battery Charge and Discharge Using the Keithley Model 2450 or 2460 SourceMeter SMU Instrument
Learn about 2400 Series SMU for Rechargeable Battery Charge and Discharge
Electrical Device Characterization
SourceMeter SMU instruments and the 4200A-SCS Parameter Analyzer are ideal for electrical device characterization because they can source and measure current and voltage. In addition to containing multiple SMU instruments, a parameter analyzer can also include a capacitance-voltage unit or pulse-measure unit. The components that can be characterized can include carbon nanostructures and devices, sensors, solar cells, organic semiconductor devices, and other structures.
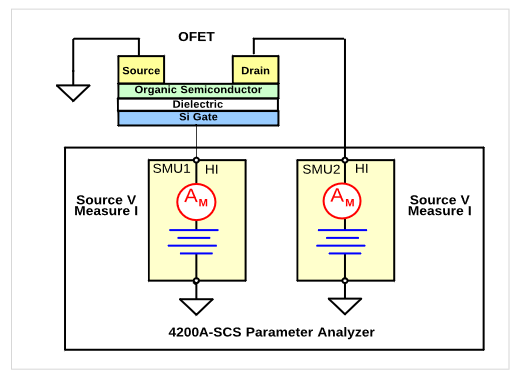
The number of SMU instruments needed for a particular application depends on the number of terminals on the device and the measurements required. In the organic FET (OFET) example shown in Figure 15, two SMU instruments are required to characterize the device. In this case, one instrument (SMU1) is connected to the Gate terminal and a second instrument (SMU2) is connected to the Drain terminal of the device. The Source terminal of the OFET is connected to common. The transfer characteristics of the OFET is determined by stepping the Gate voltage using SMU1 and sweeping the Drain voltage and measuring the Drain current using SMU2.
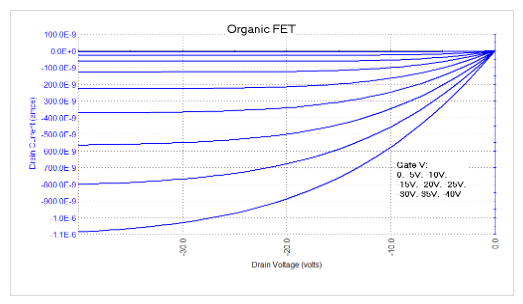
The transfer characteristics of an OFET measured and graphed by the 4200A-SCS Parameter Analyzer are shown in Figure 16.
The 4200A-SCS Parameter Analyzer offers many advantages for characterizing devices electrically. This configurable test system can simplify sensitive electrical measurements because it integrates multiple instruments into one system that includes interactive software, graphics, and analysis capabilities.
Explore 4200A-SCS Parameter Analyzer
Electrodeposition, Electroplating
Electrodeposition is the process of applying a thin film of metal to a conductive surface. This process has many applications including decorative coatings, corrosion prevention, and even nanowire and nanostructure fabrication. Traditionally, this process involved a current source connected between two electrodes, an anode and a cathode. The current drives the metal ions from the anode to the cathode as shown in Figure 17. In this simple example, a 6220 Current Source causes the Ag+ ions from the anode to be drawn to the cathode.
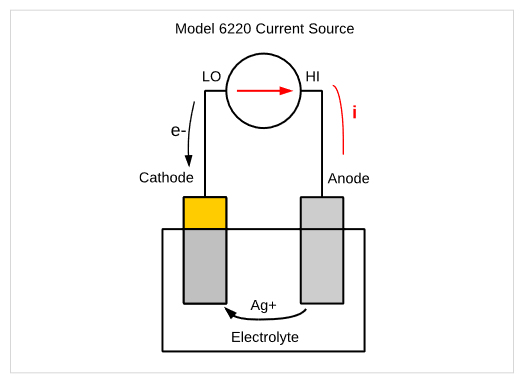
Electrodeposition may require using a constant DC current or voltage, or it may require a pulsed or stepped signal with controlled deposition time. In addition to sourcing a current or voltage, the specific application may require monitoring current or voltage. A Series 2400 or Series 2600B SourceMeter SMU instrument can control the parameters of the source automatically, as well as monitor the resulting current or potential in the circuit. Four-wire control from the instrument to the 2-electrode configuration can be used to eliminate the effects of lead resistance.
Conclusion
Keithley Instruments manufactures sensitive equipment suitable for a variety of electrochemical applications, including voltammetry, low and high resistivity measurements, battery test, potentiometry, electrodeposition, electrical device characterization, and other tests that involve sourcing and measuring current and voltage and measuring capacitance with high accuracy. Keithley instrumentation can be controlled remotely for automated test, synchronization, and timing control.
Keithley Products for Electrochemical Test Methods and Applications
| Keithley Model | Test Instrument | Description | Test Method/Application |
| 2450-EC, 2460-EC, and 2461-EC | Electrochemistry Lab Systems | 2450, 2460, or 2461 SourceMeter SMU Instrument with built in test scripts and electrochemistry translation cable. | Cyclic voltammetry, open circuit potential, potential pulse and square wave with current measurements, current pulse and square wave with potential measurements, chronoamperometry, chronopotentiometry.(Not in EC package: battery charge/discharge, device characterization, potentiometry, sensors, electrodeposition, nanotechnology, electrochemical etching, solar cells, corrosion, resistivity) |
| Series 2400 and Series 2600B | SourceMeter Source Measure Unit (SMU) instruments | SMU instruments are precision solutions for sourcing current or voltage and simultaneously measuring current, voltage and/or resistance with high speed and accuracy. | Open circuit potential, battery charge/discharge, device characterization, potentiometry, sensors, electrodeposition, nanotechnology, electrochemical etching, solar cells, corrosion, resistivity |
| 4200A-SCS | Parameter Analyzer | Modular, fully integrated parameter analyzer that performs electrical characterization of materials and devices. Intuitive software provides easy parameter extractions, test configuration and analysis. Modules include medium and high power DC, ultra-fast pulsed DC, and capacitance/impedance. | Organic semiconductor device characterization, carbon based device characterization, leakage current, sensors, solar cells, nanotechnology, resistivity, potentiometry |
| 6517B, 6514 | Electrometer | Multi-function instrument that measures voltage with high input impedance, low current (<1pA), charge, and high resistance, beyond the capabilities of a conventional digital multimeter. | Potentiometry, resistivity of insulating materials, sensors, pH measurements, ion selective electrodes |
| 6485, 6487 | Picoammeter (amperometric) | An ammeter optimized for the precise measurement of small currents. Often used in high resistance measurements. | High resistivity, leakage current, sensors |
| 6220, 6221 | Current Source (galvanostatic) | An instrument designed to output a specified current through a device under test and apply whatever voltage is required for that current. | Resistivity (with voltmeter), electrodeposition, sensors, Hall effect |
| 2182A | Nanovoltmeter | A voltmeter optimized to provide nanovolt sensitivity. Measure voltages near the theoretical limits from low source resistances. | Resistivity (with current source), very low voltage output characteristics, sensors, Hall effect |
| DMM6500, DAQ6510, DMM7510, 2000, 2750, 2010, 3706A | Digital Multimeters | Multi-function measuring instruments: DC and AC voltage and current, resistance, temperature | Resistivity, sensors, corrosion |
Find more valuable resources at TEK.COM
Copyright © Tektronix. All rights reserved. Tektronix products are covered by U.S. and foreign patents, issued and pending. Information in this publication supersedes that in all previously published material. Specification and price change privileges reserved. TEKTRONIX and TEK are registered trademarks of Tektronix, Inc. All other trade names referenced are the service marks, trademarks or registered trademarks of their respective companies.
042518 1KW-60158-1

